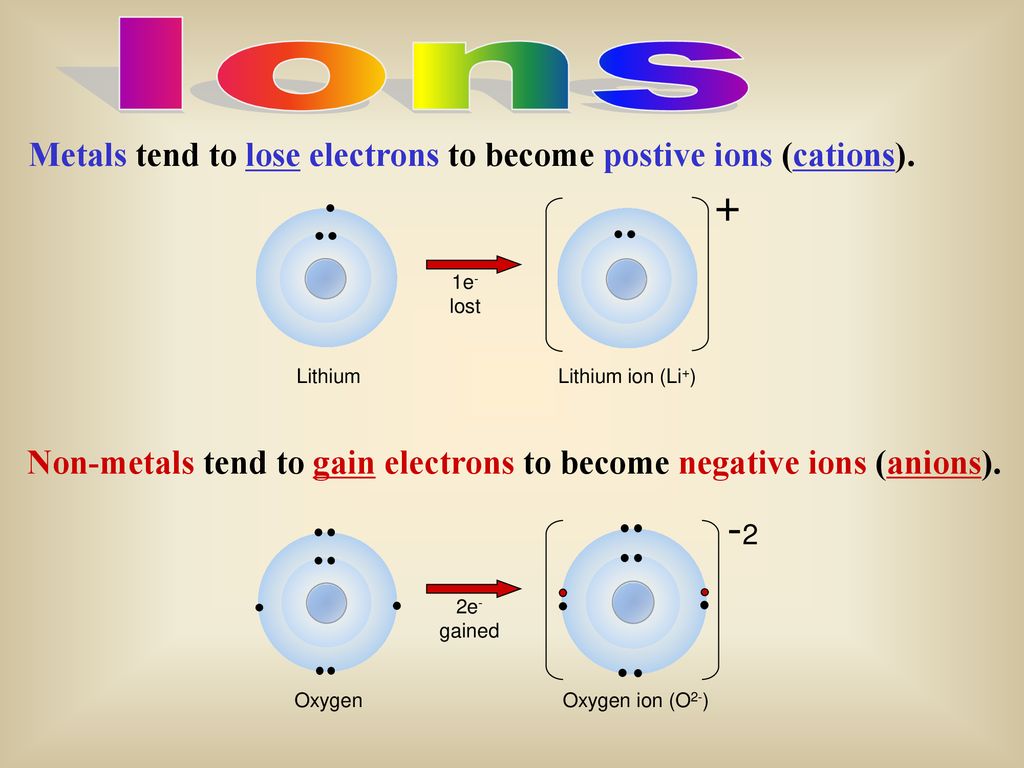
Metals Tend to Lose Electrons to Become Positive Ions
Access the answers to hundreds of The periodic table questions that are explained in a way thats easy for you to understand. Its electronegativity is 40.

What Are Ions When Atoms Gain Or Lose Electrons They Become Ions This Means They Are No Longer Neutral Unit 3 Chemistry Ions And Ionic Bonding Ppt Download
For example zinc generally loses two electrons from its 4s subshell to adopt a pseudo-noble gas configuration.
. With some exceptions those in nonmetals are fixed in place resulting in nonmetals usually being poor. Electron Configurations of Ions Transition metals also lose electrons from the valence shell first which is not the last subshell to fill according to the aufbau sequence. Those elements requiring only a few electrons to complete their valence shells and having the least quantity of inner electron shells between the positive nucleus and the valence electrons are the most electronegative.
In chemistry a nonmetal is a chemical element that generally lacks a predominance of metallic properties. Zn Zn2 2 e- Ar4s23d10 Ar3d10. Get help with your The periodic table homework.
Cant find the. They range from colorless gases like hydrogen to shiny and high melting temperature solids like boronThe electrons in nonmetals behave differently to those in metals. Metals have electronegativities less.
The most electronegative of all elements are fluorine.
How Do Metals Tend To Lose Electrons To Form Positive Ions Quora

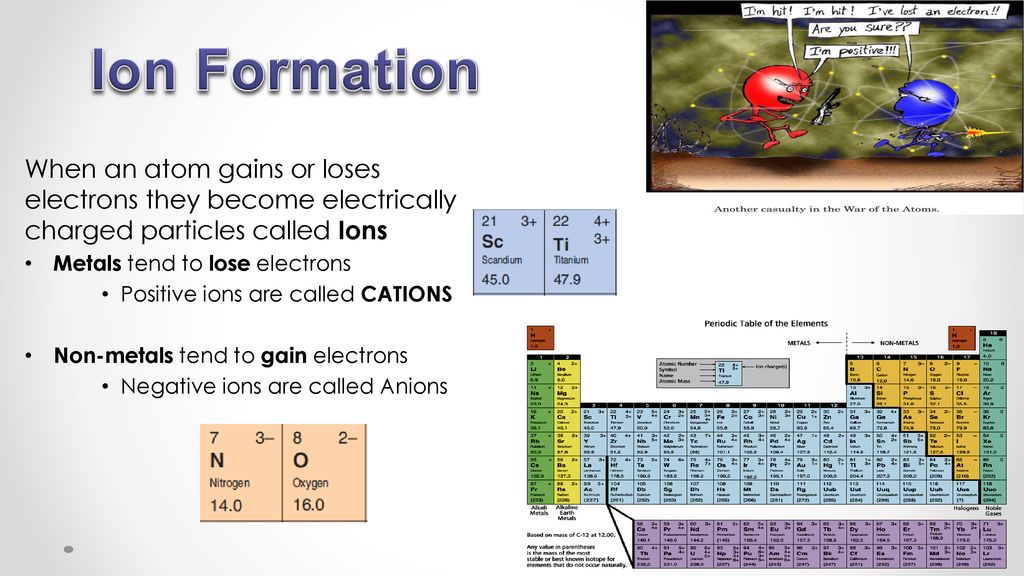
Ion Formation When An Atom Gains Or Loses Electrons They Become Electrically Charged Particles Called Ions Metals Tend To Lose Electrons Positive Ions Ppt Download

Atoms Of Metals Tend To1 Lose Electrons And Form Negative Ions2 Lose Electrons And Form Positive Brainly Com
Comments
Post a Comment